RESEARCH GROUP
Molecular signaling in muscle metabolism
Our research focus on the mechanism(s) by which physical activity promotes metabolic health. Our studies have focused on the role of the energy sensor AMP activated protein kinase in promoting adaptations in skeletal muscle to exercise training and to a single bout of exercise.
Of particular interest is the interaction at the intracellular level between exercise-induced signaling events and those induced by insulin trying to elucidate the mechanisms by which a single bout of exercise can improve muscle insulin sensitivity.
Our interest in energy metabolism is focused on the regulation of turnover and storage of both glucose and fatty acids.
Our approaches to gain such insights include invasive studies of healthy and diseased subjects in combination with mechanistic studies using animal models - including various transgenic approaches.
We seek to adapt state of art technologies in our analyses-tool box - including various omics platforms. Further, we seek to gain insight at the single muscle fiber level using both biochemistry and imaging techniques.
In collaborations with various clinical laboratories and pharma industries we aim to translate our findings into relevance for patients and society.
We believe that by illuminating the mechanism by which physical activity promotes health we will be able pointing toward pharmacological interventions eliciting the same healthy beneficial effects as exercise.
Research projects
Model systems using cultured primary human myotubes and muscle cell lines derived from rodents all suffer from lack of differentiation and do not possess many of the specialized features found in mature human skeletal muscle. Hence, exploring the possibility for culturing skeletal muscle from the relatively easy obtainable needle muscle biopsy holds great potential for both basic and pharmaceutical research.
Research within muscle metabolism has increased substantially recent years due to the well-established positive effects of physical activity and exercise on overall health including a decreased risk of developing cardiovascular diseases, hypertension, cognitive dysfunction, certain types of cancer, and type 2 diabetes.
Unfortunately, good model systems for the study of human skeletal muscle are lacking. Model systems using cultured primary myotubes (originating from skeletal muscle satellite cells or embryonic stem cells) and muscle cell lines derived from rodents (mouse C2C12 and rat L6) all suffer from lack of differentiation and do not possess many of the specialized features found in mature skeletal muscle. Therefore, we want to explore the possibility for culturing muscle fibers obtained from Bergström needle muscle biopsies.
Such tissue samples are relatively easy obtainable from subjects, and can be obtained repeatedly. We foresee that this model system will diminish translational losses between species (cell → animal → human findings). Furthermore, we will be able to relate findings from the ex vivo model to both the in vivo skeletal muscle and whole body metabolism of the same individual from which the skeletal muscle tissue is obtained.
Funded by
Period: 2017 - 2023.
Contact
Assistant professor Rasmus Kjøbsted
Professor Jørgen Wojtaszewski
Recently, we provided evidence to support that exercise and contraction increase insulin sensitivity of mouse skeletal muscle by an AMPK-dependent mechanism. By manipulating AMPK activity in human skeletal muscle using ischemia alone or in combination with exercise we will investigate the possible role of AMPK in regulating muscle insulin sensitivity in man.
Improvement of skeletal muscle insulin resistance is one of the corner stones for the treatment of type 2 diabetes (T2D), as this tissue is responsible for approximately 75% of total insulin-stimulated glucose disposal.
Acute and chronic exercise is known to improve skeletal muscle insulin sensitivity via intracellular signaling events, including activation of the AMPK signaling pathway.
Exercise-induced activation of AMPK signaling in skeletal muscle is regulated in a time- and intensity-dependent manner, indicating that intense exercise is preferable for the treatment of T2D. However, intense exercise can be a risk factor of complications in T2D patients, thus new treatment regimens with lower risk are needed.
Interestingly, ischemia has also been shown to increase AMPK activation in the target tissue. Therefore, as a low risk treatment, ischemia has the likely potential to improve skeletal muscle insulin sensitivity via AMPK.
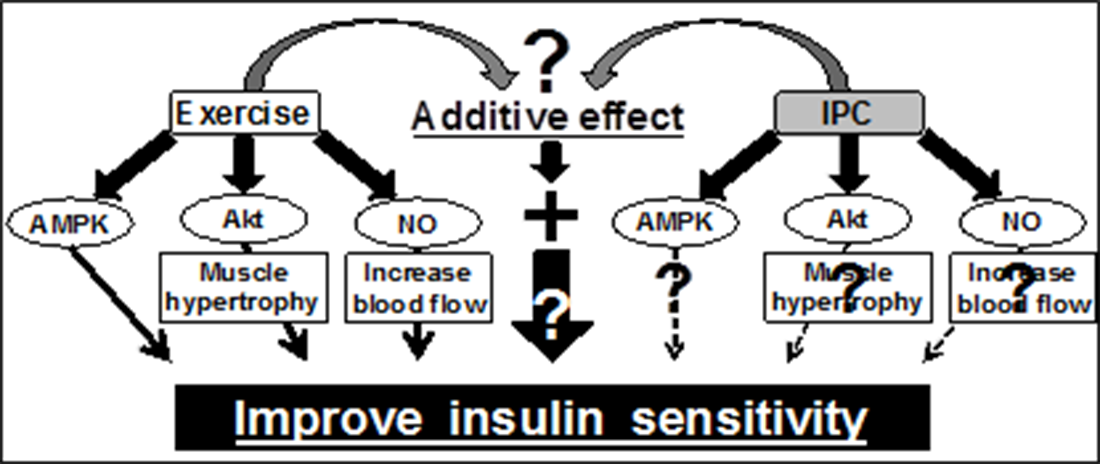 Furthermore, combining ischemia and low intense exercise may additively activate these signaling pathways, which leads to further improvement of muscle insulin sensitivity. However, the effect of ischemia on muscle glucose metabolism is unknown and therefore the current project seeks to investigate whether ischemia affects AMPK activity and glucose metabolism at the resting and exercising states in human skeletal muscle.
Furthermore, combining ischemia and low intense exercise may additively activate these signaling pathways, which leads to further improvement of muscle insulin sensitivity. However, the effect of ischemia on muscle glucose metabolism is unknown and therefore the current project seeks to investigate whether ischemia affects AMPK activity and glucose metabolism at the resting and exercising states in human skeletal muscle.
Funded by
Novo Nordisk Foundation (NNF)
Period: 2018 - 2022.
Contact
Assistant professor Rasmus Kjøbsted
Professor Jørgen Wojtaszewski
A single bout of exercise increases glucose uptake and increases insulin sensitivity in recovery from exercise. Here we aim to study the underlying molecular mechanisms by use of phosphoproteomics in muscle biopsies from healthy subjects as well as insulin resistant subjects.
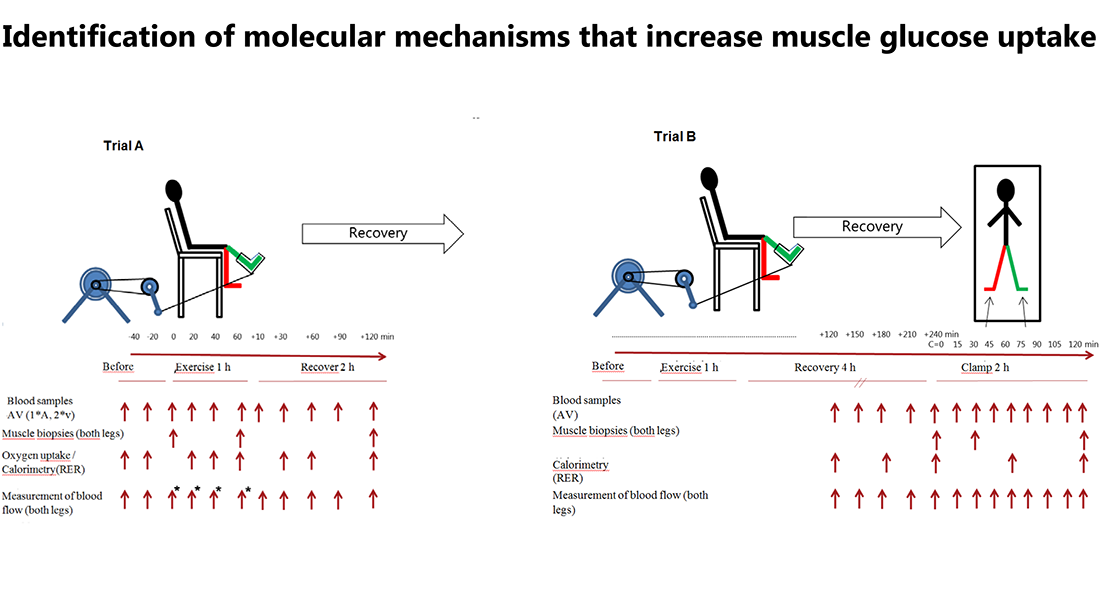
Proposal
The purpose of this study is to do a profound analysis of the molecular changes that take place during physical activity in muscles and lead to increased glucose uptake in the muscles during exercise and lead to increased sensitivity to insulin during the time period after work in both healthy persons and in person with insulin resistance.
10 healthy subjects and 10 subjects with insulin resistance will perform one-legged exercise at two different trials (trial A and B).
Trial A consists of 1 hour of one-legged exercise with muscle biopsies taken from both legs before, immediately after exercise and following 3 hours recovery.
Trial B consists of one-legged exercise where insulin infusion will be initiated 4 hours after exercise. Basal and insulin-stimulated biopsies during trial B will be collected before, following 30 min insulin infusion as well as after 120 min insulin infusion.
More specifically we aim, in cooperation with senior scientists Christian Pehmøller at Pfizer Inc. in Boston and Professor David James from Sidney University, Australia, who is a leading expert within MS-based omics analyses, to investigate:
- Changes in glucose uptake in the muscle during exercise and subsequent stimulation with insulin will be examined by measuring the amount and posttranslational modification of proteins in muscles.
- Changes in the protein content and modification in blood plasma as a result of physical work and during the subsequent insulin stimulation.
Funded by
Pfizer Inc. (Boston) and a part of a collaboration project between Copenhagen University, University of Sydney and Pfizer Inc..
Period: 2018 - 2023.
Contact
Professor Jørgen Wojtaszewski
TBC1 domain family member 4 (TBC1D4) is a key intermediate in the process of insulin-stimulated glucose uptake in skeletal muscle and mutations in human TBC1D4 have been associated with a higher risk of developing type 2 diabetes. Thus, identifying interacting proteins of TBC1D4 may represent novel targets for future pharmaceutical strategies aimed at preventing/treating type 2 diabetes.
The purpose of this study is to investigate potential interacting partners of the GTPase-activating protein TBC1D4 (also known as AS160) in human skeletal muscle during basal and insulin-stimulated conditions (2 h hyperinsulinemic euglycemic clamp).
TBC1D4 accelerates the GTP-hydrolysis of Rab proteins and is involved in the translocation of GLUT4 to the plasma membrane during insulin stimulation. Thus, any potential interacting partner of TBC1D4 in skeletal muscle may also be involved in regulating glucose uptake.
In the search for potential interacting partners of TBC1D4, we plan to immunoprecipitate TBC1D4 from obtained human skeletal muscle samples for subsequent proteomic analyses. We suspect an interaction of scaffold-proteins, but the exact nature and role of these are unknown.
Furthermore, this discovery-based LC-MS/MS approach opens the door for identification of novel GAP binding proteins important for localization and regulation of TBC1D4. Identification of TB1CD4 interacting partners holds great promise for illuminating novel proteins involved in the regulation of muscle glucose uptake that may serve as targets for pharmacological interventions aimed at alleviating muscle insulin resistance.
Funded by
The Danish Council for Independent Research in Medical Sciences
Period: 2017 - 2021.
Contact
Assistant professor Rasmus Kjøbsted
Professor Jørgen Wojtaszewski
Current evidence suggests that the insulin-sensitizing effect of acute exercise/contraction on muscle glucose uptake depend on an AMPK-TBC1D4 signaling axis in skeletal muscle. Using transgenic TBC1D4 mice, we will investigate the possible role of TBC1D4 in regulating muscle insulin sensitivity after AMPK activating stimuli (exercise, contraction, and AMPK agonists).

The Rab GTPase-activating protein TBC1D4 has been shown to be important for insulin-stimulated glucose uptake due to its regulatory effect on the GLUT4 translocation process.
TBC1D4 is regulated via phosphorylation by upstream kinases Akt (insulin-activated) and AMPK (exercise-activated) and has emerged as a potential candidate for mediating the enhanced effect of prior exercise on muscle insulin sensitivity. This is supported by observations in human in which increased phosphorylation of TBC1D4 coincides with enhanced insulin sensitivity in prior exercised muscle.
Recently, we provided evidence to support a direct role of AMPK in regulating muscle insulin sensitivity possibly through TBC1D4. However, causality remains to be established between improvements in insulin-stimulated muscle glucose uptake and regulation of TBC1D4 signaling following acute exercise, contraction and AMPK agonists. Using both TBC1D4 knockout and knockin mouse models, we will investigate the potential role of TBC1D4 in regulating muscle insulin sensitivity for glucose uptake.
A better understanding of the underlying mechanism regulating the health benefits of exercise is important to effectively prescribe exercise in general but also to utilize the large pharmacological potential of "exercise mimetics" for physically disabled or the many for whom physical activity is not attractive.
Funded by
The Danish Council for Independent Research in Medical Sciences
Period: 2016 - 2022.
Contact
Assistant professor Rasmus Kjøbsted
Professor Jørgen Wojtaszewski
A common nonsense variant in the gene TBC1D4 (p.Arg684Ter) were recently discovered in the Greenlandic Inuit population. This isoform is predominantly expressed in skeletal muscle, and TBC1D4 protein is markedly decreased in muscle of homozygotes carriers. Homozygous carriers of this variant have impaired glucose tolerance and a 10-fold higher risk of T2D. We perform a comprehensive metabolic phenotyping of adult Inuit’s carrying zero (controls) or two alleles (cases) of the variant.
Proposal
We will investigate whether regulation of TBC1D4 is pivotal in regulating glucose uptake during exercise, during physiological insulin stimulation, and for the ability of an acute bout of exercise (one-legged knee extensor exercise) to improve insulin sensitivity in human skeletal muscle.
The project will be based on well-controlled highly invasive studies of individuals with zero or two alleles of the TBC1D4 variant. Thus, applying A-V balance technique across the legs during exercise and during euglycemic hyperinsulinemic clamp conditions (in the prior rested and the prior exercise state) allows estimation of glucose uptake during exercise and during insulin stimulation in prior exercised and rested legs, respectively.
These measures will be combined with multiple muscle biopsies allowing for comprehensive muscle specific analyses; including deep RNA sequencing, metabolomics, (phosphor) proteomic and quantitative and detailed evaluation of canonical intermediates in insulin- and exercise-induced signaling pathways.
The project will provide us with unique insight to the role of TBC1D4 in glucose metabolism in human. Further, the insights will create a valid foundation for development of genotype guided interventions with the potential for improvement of the prognosis of affected individuals.
Funded by
The Danish Council for Independent Research in Medical Sciences
Period: 2014 - 2022.
Contact
Professor Jørgen Wojtaszewski
Members of research group
| Name | Title | Phone | |
|---|---|---|---|
| Jesper Bratz Birk | Academic Staff | +4535321613 | |
| Johan Dejgaard Onslev | Postdoc | ||
| Jørgen Wojtaszewski | Professor, Head of Section | +4535321625 | |
| Magnus Romme Leandersson | PhD Fellow | +4535320766 | |
| Nicolai Stevns Henriksen | PhD Fellow | +4535325071 | |
| Rasmus Kjøbsted | Associate Professor | +4535321764 |