RESEARCH GROUP
Molecular signaling in muscle metabolism
Our research focus on the mechanism(s) by which physical activity promotes metabolic health. Our studies have focused on the role of the energy sensor AMP activated protein kinase in promoting adaptations in skeletal muscle to exercise training and to a single bout of exercise.
Of particular interest is the interaction at the intracellular level between exercise-induced signaling events and those induced by insulin trying to elucidate the mechanisms by which a single bout of exercise can improve muscle insulin sensitivity.
Our interest in energy metabolism is focused on the regulation of turnover and storage of both glucose and fatty acids.
Our approaches to gain such insights include invasive studies of healthy and diseased subjects in combination with mechanistic studies using animal models - including various transgenic approaches.
We seek to adapt state of art technologies in our analyses-tool box - including various omics platforms. Further, we seek to gain insight at the single muscle fiber level using both biochemistry and imaging techniques.
In collaborations with various clinical laboratories and pharma industries we aim to translate our findings into relevance for patients and society.
We believe that by illuminating the mechanism by which physical activity promotes health we will be able pointing toward pharmacological interventions eliciting the same healthy beneficial effects as exercise.
Research projects
 We have recently developed the physiological and bioinformatic approach personalized phosphoproteomics to identify novel molecular mechanisms of insulin stimulated glucose uptake into muscle.
We have recently developed the physiological and bioinformatic approach personalized phosphoproteomics to identify novel molecular mechanisms of insulin stimulated glucose uptake into muscle.
Here, we aim to utilize the same approach to identify the regulatory mechanisms of glucose uptake, lactate release, and glycogen breakdown from muscle during exercise.
The study will include data from two similar, but separate cohorts, to strengthen the bioinformatic analysis.
Proposal
The purpose of this study is to elucidate novel molecular mechanisms regulating muscle glucose metabolism, in the form of glucose uptake, lactate release and glycogen breakdown, during exercise.
Approximately 50 subjects will be included and divided into two different cohorts. The two cohorts will serve multiple purposes, some of them not listed here. All subjects will, however, perform 1 hour of one-legged knee-extensor exercise. Prior to exercise, femoral artery and venous catheters will be inserted to dynamically determine glucose uptake and lactate release of each leg over the exercise bout.
Muscle biopsies will be collected from the rested and the exercising leg immediately after the exercise bout. Pieces of the muscle samples will be subjected to biochemical analysis to determine glycogen content. Other pieces of the muscle samples will be analyzed by phosphoproteomics analysis to determine the dynamic molecular changes that occur in response to the exercise bout.
Subsequently, we will identify the top molecular changes resembling the variation in glucose uptake, lactate release and glycogen breakdown across both study cohorts (the personalized phosphoproteomics approach).
In collaboration with Associate Professor Sean Humphrey from Murdoch Children’s Research Institute in Melbourne, Australia, who is a leading expert within MS-based omics analyses.
Funded by
Danish Diabetes and Endocrine Academy
Novo Nordisk Foundation's Distinguished program
Period: 2021 - 2027
Contact
Professor Jørgen Wojtaszewski
PhD Student Magnus Romme Leandersson
Recent human studies from our group indicate a promising role of the protein mTORC1 in regulating muscle insulin sensitivity, specifically in the recovery period following exercise.
Here we utilize the drug rapamycin, a specific inhibitor of mTORC1, as a tool to assess the role of mTORC1 in muscle insulin sensitivity.
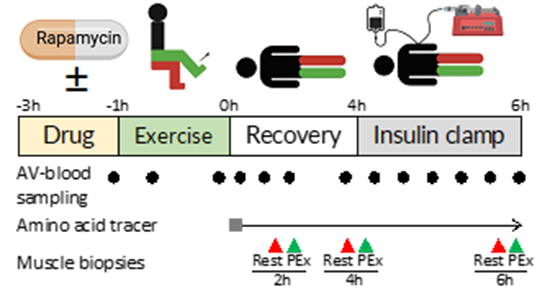
The study includes healthy subjects ingesting rapamycin, performing exercise, and subsequently undergoing an insulin clamp.
Proposal
The purpose of this study is to elucidate the role of mTORC1 in regulation of muscle insulin sensitivity in the period following exercise. Muscle insulin sensitivity will be assessed from glucose uptake obtained by arteriovenous blood sampling, and from protein synthesis and breakdown obtained from amino acid tracer infusion.
12-14 healthy subjects will be included in a crossover blinded study. On each study day, the subject will ingest rapamycin or placebo, perform one-legged knee-extensor exercise and subsequently undergo an insulin clamp. Femoral artery and venous catheters will be inserted to dynamically determine glucose uptake of each leg over the study day.
An amino acid tracer will be infused to determine protein metabolism (synthesis and breakdown) in the recovery period from exercise and during the insulin clamp. Muscle biopsies will be collected before and after the insulin clamp. These muscle samples will be analyzed by phosphoproteomics analysis to determine the dynamic molecular changes that occur in response to the drug rapamycin.
In collaboration with Associate Professor Sean Humphrey from Murdoch Children’s Research Institute in Melbourne, Australia, who is a leading expert within MS-based omics analyses.
Funded by
Danish Diabetes and Endocrine Academy
Novo Nordisk Foundation's Distinguished program
Period: 2021 - 2027
Contact
Professor Jørgen Wojtaszewski
PhD Student Magnus Romme Leandersson
AMPK and mTORC1 are two central kinases regulating skeletal muscle metabolism, and regulation of these kinases is of major interest in the context of skeletal muscle insulin resistance and type 2 diabetes, skeletal muscle mass and sarcopenia, and adaptation to exercise training.
AMPK-mediated inhibition of mTORC1 is described extensively in the literature, but mTORC1 has only recently been described to inhibit AMPK. This has been demonstrated in various cells (including yeast and mouse embryonic fibroblasts) and occurs via mTORC1-mediated phosphorylation of AMPK on Ser345. The relevance of this mechanism in skeletal muscle cells remains to be elucidated.
Furthermore, phosphorylation of Ser377 on AMPK has been observed to correlate positively with insulin-stimulated glucose uptake in human skeletal muscle, and this phosphorylation also occurs downstream of mTORC1.
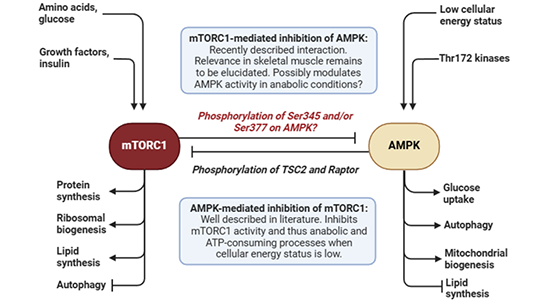
This project seeks to expand upon these mTORC1-mediated phosphorylations, specifically in skeletal muscle of mouse and possibly human. We have performed experiments in mouse skeletal muscle demonstrating that both phosphorylations occur in response to mTORC1 stimuli, and that both phosphorylations are abrogated with administration of mTORC1 inhibitors.
Current and future experiments seek to further elucidate the physiological roles of this interaction between mTORC1 and AMPK in skeletal muscle by utilizing approaches including:
(1) various exercise modalities and protocols;
(2) administration of nutrients, insulin, and/or mTORC1 inhibitors; and
(3) the generation of a Ser345Ala and Ser377Ala phospho-resistant knockIn mouse model.
If mTORC1 indeed inhibits AMPK activity/function in skeletal muscle, it may pose therapeutic implications for targeting mTORC1 and thus possibly positively regulate metabolic health.
Funded by
The University of Copenhagen and the Novo Nordisk Foundation.
Contact
Professor Jørgen Wojtaszewski
Associate Professor Rasmus Kjøbsted
Research Assistant Magnus Zankel
 Background
Background
Type 2 diabetes (T2D) is one of the most prevalent diseases in the western world, yet the cellular and molecular signals driving insulin resistance — a key contributor to T2D — remain poorly understood.
Current diagnostic tools often fail to capture the complexity of insulin resistance, which involves multiple steps in glucose uptake and utilization. Moreover, individual differences in genetic predisposition and response to lifestyle factors further complicate our understanding of this condition.
Proposal
Signature aims to uncover the molecular mechanisms underlying insulin resistance by identifying subphenotypes at both phenotypic and molecular levels in a healthy population of 80 males and females aged 25–55 years.
Using state-of-the-art techniques, we will assess various aspects of metabolism in depth and hereby displaying the ground state heterogeneity among individuals. This will be combined with analyses of proteins, metabolites, and genes within metabolic relevant tissues such as blood, muscle and fat to explore individual profiling.
In addition, participants will be examined following two short-term interventions aiming to induce temporal insulin resistance i.e.: Reduced physical activity or a 3-day hypercaloric diet.
Significance
The project hypothesizes that insulin resistance is influenced by distinct genetic variants, leading to varied molecular causes and responses to lifestyle interventions.
By identifying these mechanisms, Signature aims to inform precision medicine approaches for preventing and managing insulin resistance/T2D.
Additionally, insights from this research could guide the development of targeted therapies.
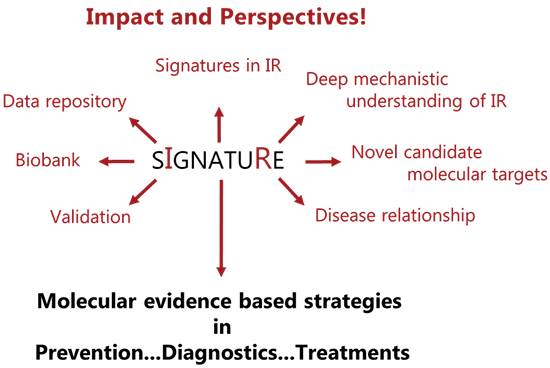
Collaborations
The project is conducted at the Department of Nutrition, Exercise and Sports, University of Copenhagen, in collaboration with Professor Claudia Langenberg at Queen Mary University of London in London, UK and Professor David James at the University of Sydney, Australia.
Funded by
The project is funded by the Novo Nordisk Foundation's Challenge Program.
Period: June 2025 – 2031.
Contact
Professor Jørgen Wojtaszewski
The TBC1D4 protein is essential for insulin-stimulated glucose uptake. A common TBC1D4 variant (p.Arg684Ter) is found among Greenlandic Inuit, and homozygous carriers lack TBC1D4 protein in skeletal muscle.
This results in decreased glucose tolerance and a 10-fold higher risk of developing type 2 diabetes.
Currently, treatment options for these variant carriers are limited. Here, we aim to explore the role of TBC1D4 in exercise-regulated glucose metabolism.
Proposal
We conducted a 6-week high-intensity interval training (HIIT) intervention study in Nuuk, Greenland, to investigate the effect of regular exercise training on glucose tolerance and molecular mechanisms in TBC1D4 variant carriers.
The participants included Greenlandic homozygous carriers of the variant and matched non-carriers. Before and after the HIIT intervention an oral glucose tolerance test and 14-days continuous glucose monitoring were performed to investigate the effect on glucose tolerance. Additionally, skeletal muscle and fat biopsies were obtained to analyse exercise-induced molecular signalling.
If exercise improves glucose tolerance in the variant carriers, it could provide a non-pharmacological approach to reduce type 2 diabetes risk. Furthermore, identifying molecular mechanisms underpinning the beneficial effects of exercise may lead to novel pharmacological therapies for individuals unable to engage in physical activity.
Funded by
The project is a collaboration between Steno Diabetes Center Greenland and the University of Copenhagen. The project has received funding from the Greenland Research Council.
Period: 2023-2026.
Contact
Professor Jørgen Wojtaszewski
Associate Professor Rasmus Kjøbsted
Research Assistant Cecilie With
Model systems using cultured primary human myotubes and muscle cell lines derived from rodents all suffer from lack of differentiation and do not possess many of the specialized features found in mature human skeletal muscle. Hence, exploring the possibility for culturing skeletal muscle from the relatively easy obtainable needle muscle biopsy holds great potential for both basic and pharmaceutical research.
Research within muscle metabolism has increased substantially recent years due to the well-established positive effects of physical activity and exercise on overall health including a decreased risk of developing cardiovascular diseases, hypertension, cognitive dysfunction, certain types of cancer, and type 2 diabetes.
Unfortunately, good model systems for the study of human skeletal muscle are lacking. Model systems using cultured primary myotubes (originating from skeletal muscle satellite cells or embryonic stem cells) and muscle cell lines derived from rodents (mouse C2C12 and rat L6) all suffer from lack of differentiation and do not possess many of the specialized features found in mature skeletal muscle. Therefore, we want to explore the possibility for culturing muscle fibers obtained from Bergström needle muscle biopsies.
Such tissue samples are relatively easy obtainable from subjects, and can be obtained repeatedly. We foresee that this model system will diminish translational losses between species (cell → animal → human findings). Furthermore, we will be able to relate findings from the ex vivo model to both the in vivo skeletal muscle and whole body metabolism of the same individual from which the skeletal muscle tissue is obtained.
Funded by
Period: 2017 - 2026.
Contact
Assistant professor Rasmus Kjøbsted
Professor Jørgen Wojtaszewski
Recently, we provided evidence to support that exercise and contraction increase insulin sensitivity of mouse skeletal muscle by an AMPK-dependent mechanism. By manipulating AMPK activity in human skeletal muscle using ischemia alone or in combination with exercise we will investigate the possible role of AMPK in regulating muscle insulin sensitivity in man.
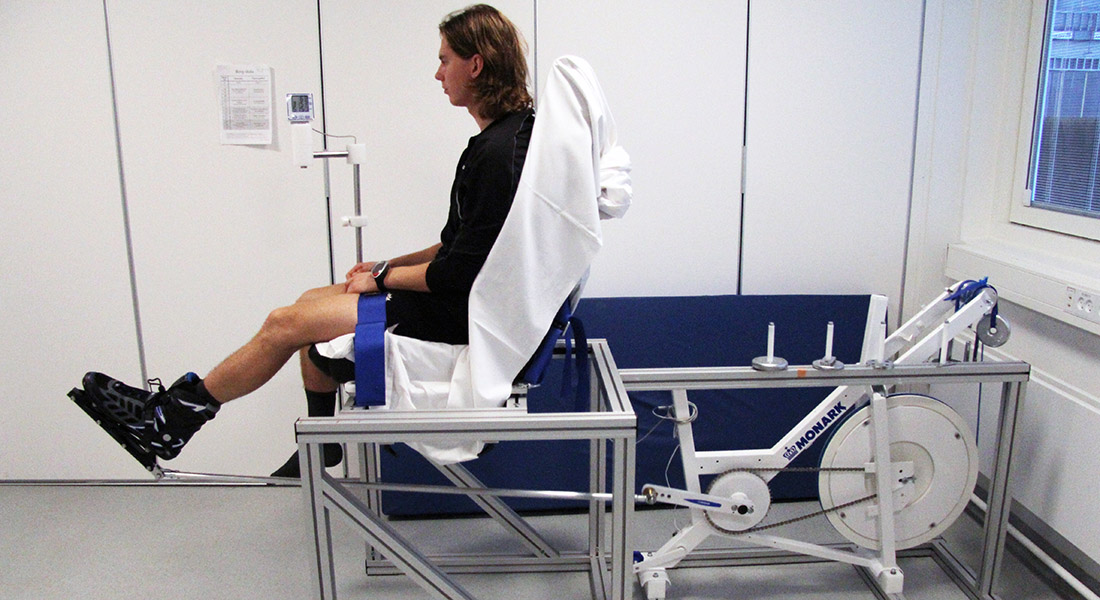
Improvement of skeletal muscle insulin resistance is one of the corner stones for the treatment of type 2 diabetes (T2D), as this tissue is responsible for approximately 75% of total insulin-stimulated glucose disposal.
Acute and chronic exercise is known to improve skeletal muscle insulin sensitivity via intracellular signaling events, including activation of the AMPK signaling pathway.
Exercise-induced activation of AMPK signaling in skeletal muscle is regulated in a time- and intensity-dependent manner, indicating that intense exercise is preferable for the treatment of T2D. However, intense exercise can be a risk factor of complications in T2D patients, thus new treatment regimens with lower risk are needed.
Interestingly, ischemia has also been shown to increase AMPK activation in the target tissue. Therefore, as a low risk treatment, ischemia has the likely potential to improve skeletal muscle insulin sensitivity via AMPK.
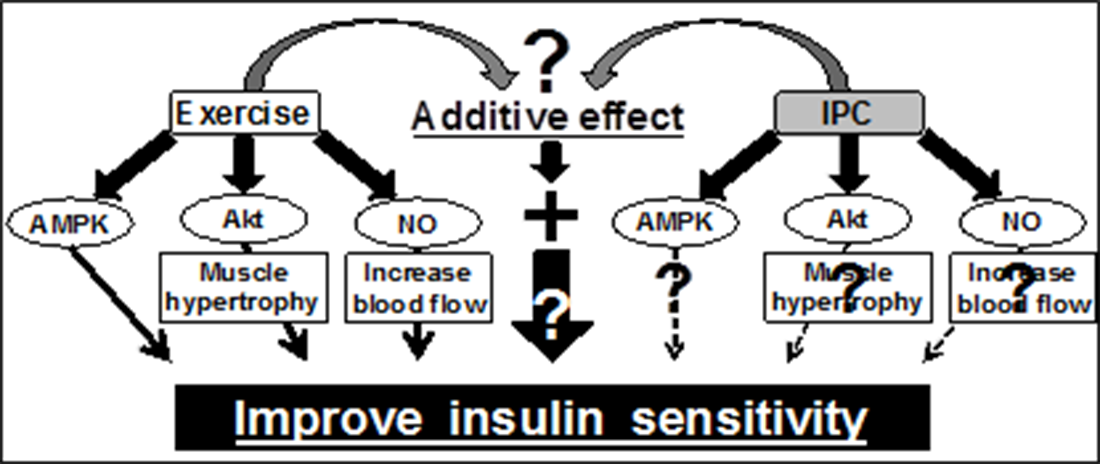 Furthermore, combining ischemia and low intense exercise may additively activate these signaling pathways, which leads to further improvement of muscle insulin sensitivity. However, the effect of ischemia on muscle glucose metabolism is unknown and therefore the current project seeks to investigate whether ischemia affects AMPK activity and glucose metabolism at the resting and exercising states in human skeletal muscle.
Furthermore, combining ischemia and low intense exercise may additively activate these signaling pathways, which leads to further improvement of muscle insulin sensitivity. However, the effect of ischemia on muscle glucose metabolism is unknown and therefore the current project seeks to investigate whether ischemia affects AMPK activity and glucose metabolism at the resting and exercising states in human skeletal muscle.
Funded by
Novo Nordisk Foundation (NNF)
Period: 2018 - 2025.
Contact
Assistant professor Rasmus Kjøbsted
Professor Jørgen Wojtaszewski
A single bout of exercise increases glucose uptake and increases insulin sensitivity in recovery from exercise. Here we aim to study the underlying molecular mechanisms by use of phosphoproteomics in muscle biopsies from healthy subjects as well as insulin resistant subjects.
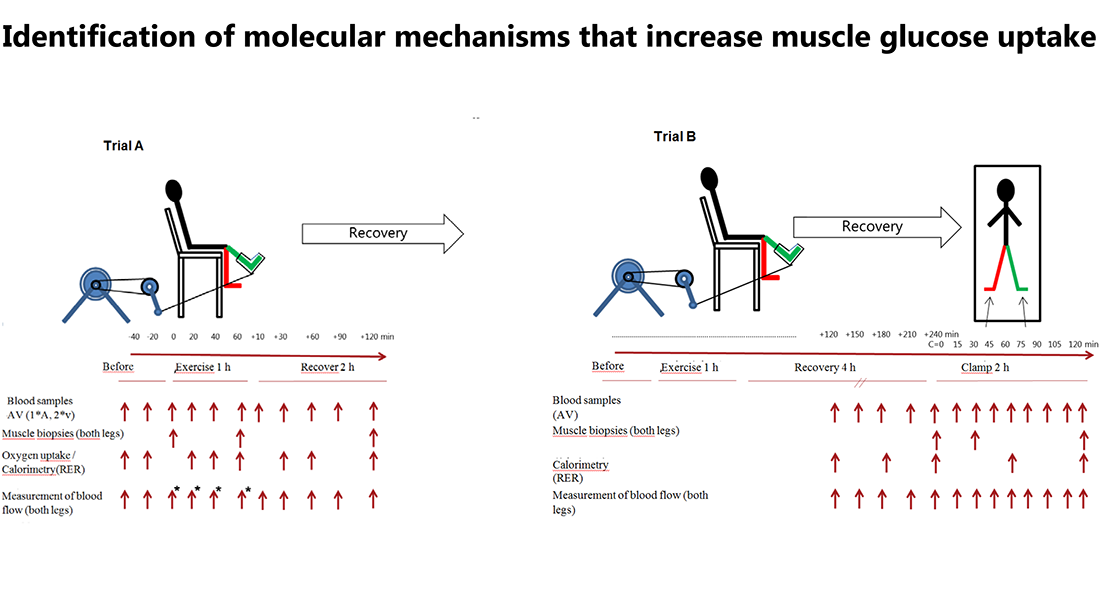
Proposal
The purpose of this study is to do a profound analysis of the molecular changes that take place during physical activity in muscles and lead to increased glucose uptake in the muscles during exercise and lead to increased sensitivity to insulin during the time period after work in both healthy persons and in person with insulin resistance.
10 healthy subjects and 10 subjects with insulin resistance will perform one-legged exercise at two different trials (trial A and B).
Trial A consists of 1 hour of one-legged exercise with muscle biopsies taken from both legs before, immediately after exercise and following 3 hours recovery.
Trial B consists of one-legged exercise where insulin infusion will be initiated 4 hours after exercise. Basal and insulin-stimulated biopsies during trial B will be collected before, following 30 min insulin infusion as well as after 120 min insulin infusion.
More specifically we aim, in cooperation with senior scientists Christian Pehmøller at Pfizer Inc. in Boston and Professor David James from Sidney University, Australia, who is a leading expert within MS-based omics analyses, to investigate:
- Changes in glucose uptake in the muscle during exercise and subsequent stimulation with insulin will be examined by measuring the amount and posttranslational modification of proteins in muscles.
- Changes in the protein content and modification in blood plasma as a result of physical work and during the subsequent insulin stimulation.
Funded by
Pfizer Inc. (Boston) and a part of a collaboration project between Copenhagen University, University of Sydney and Pfizer Inc..
Period: 2018 - 2027.
Contact
Professor Jørgen Wojtaszewski
Members of research group
| Name | Title | Phone | |
|---|---|---|---|
| Amalie Pihl Frøkiær | Research Assistant | +4535326524 | |
| Cecilie With | Research Assistant | ||
| Jesper Bratz Birk | Academic Staff | +4535321613 | |
| Johan Dejgaard Onslev | Postdoc | ||
| Jørgen Wojtaszewski | Professor, Head of Section | +4535321625 | |
| Magnus Romme Leandersson | PhD Fellow | +4535320766 | |
| Nicolai Stevns Henriksen | PhD Fellow | +4535325071 | |
| Rasmus Kjøbsted | Associate Professor | +4535321764 |